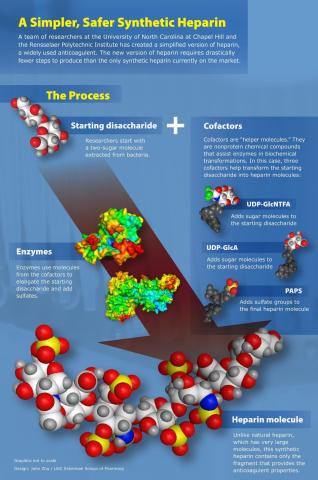
Heparin, an anticoagulant, was first used in 1916 - one of the few drugs still in use which predates the establishment of the FDA. Heparin is a long, highly negatively charged polysaccharide known as a glycosaminoglycan, or GAG. While the naturally occurring polysaccharide is quite long, the anticoagulant properties of heparin are due to a short, five saccharide section of the molecule, found in only a third of the long heparin chain. A synthetic analog of this pentasaccharide sequence, Fondaparinux, can act as a substitute for natural heparin under certain conditions. Our lab, together with Dr. Liu of the University of North Carolina at Chapel Hill, has synthesized two analogs of Fondaparinux with anticoagulant activity using a chemoenzymatic approach.
One of the advantages of these new, short, synthetic heparins is that they can be synthesized in 10 to 12 steps (vs. Fondaparinux, over 50 steps!) in high yields. This is because they are made chemoenzymatically - made using special chemical cofactors and specialized enzymes. Because enzymes are very specific in how they link molecules together, many synthetic steps can be skipped or can be done at the same time. Here, heparin and heparan sulfate biosynthetic enzymes were used to transform a simple disaccharide isolated from a bacteria into two synthetic ultra-low molecular weight heparins. Making heparin synthetically will help ensure that this important drug is chemically pure.
